Abstract
INTRODUCTION
The Graft- versus -Leukemia (G v L) effect in allogeneic stem cell transplantation (allo-SCT) is thought to be mediated largely by alloreactive T-cells which recognise minor histocompatibility antigens (mHAg). HA-1 is a potent, immunodominant mHAg which is presented by HLA-A2 and expressed selectively by hematopoietic cells. We hypothesized that immunization of HA-1- stem cell donors with an HA-1 vaccine prior to lymphocyte donation would generate HA-1-specific cytotoxic T lymphocytes (CTL) which, after adoptive transfer to the transplant recipient as donor lymphocyte infusions (DLI), may provide a potent anti-leukemia effect in HA-1+ leukemia patients without increasing the risk of Graft- versus -Host disease. Furthermore, this may allow HA-1-specific T cells to be selected and purified for targeted immunotherapy. Having previously shown that the vaccination of HLA-A2+ transgenic mice with the DNA construct (pDOM-HA-1) encoding the HLA-A2-restricted CTL-epitope, VLHDDLLEA (VLH) from HA-1, induces functional T cell responses to HA-1, we conducted a first-in-man Phase I dose-escalation trial in healthy immunocompetent HLA-A2+ and HA-1- males using the pDOM-HA-1 vaccine and subsequently boosting with a modified vaccinia Ankara virus (MVA) encoding the VLH epitope (MVA-HA-1).
PARTICIPANTS AND METHODS
The primary endpoint was vaccine safety and establishment of an optimal dose for phase II evaluation. Secondary outcomes included assessments of the kinetics and magnitude of HA-1-specific T-cell responses which were assessed weekly up to 3 months and then at months 6 and 12. 86 healthy male volunteers were screened for the trial, 20 of whom were the correct genotype. 9 participants were enrolled, median age being 45 years (range 27 - 69). Participants were to receive escalated doses of the vaccine regimen (1mg pDOM-HA-1 x 2 cycles + MVA boost (cohort 1); 1mg pDOM-HA-1 x 3 cycles + MVA boost (cohort 2), (2mg pDOM-HA-1 x 2 cycles + MVA boost (cohort 3); 2mg pDOM-HA-1 x 3 cycles + MVA boost (cohort 4). Vaccines were administered every 3 weeks. Participants were assessed for dose-limiting-toxicity (DLT) using the Toxicity Grading Scale for Healthy Volunteers Enrolled in Vaccine Trials.
RESULTS
3 participants completed the first vaccine schedule in Cohort 1 with 2 grade 2 DLTs (myalgia and headache observed in 1 participant). The observed events were not considered to be of clinical significance and the definition of a DLT was amended prior to further participants being recruited. Cohort 1 was expanded to recruit an additional 3 participants with no further DLTs observed. 3 participants received Cohort 2 with no DLTs observed. No serious adverse events were observed in any participants. The most common non-hematological toxicities were hypertension, hypermagnesemia, and injection site reactions. The most common hematological toxicities were increased monocytes, neutropenia and leucopenia. All adverse events resolved shortly after vaccine administration. The trial experienced recruitment challenges and was closed to recruitment after the completion of Cohort 2.
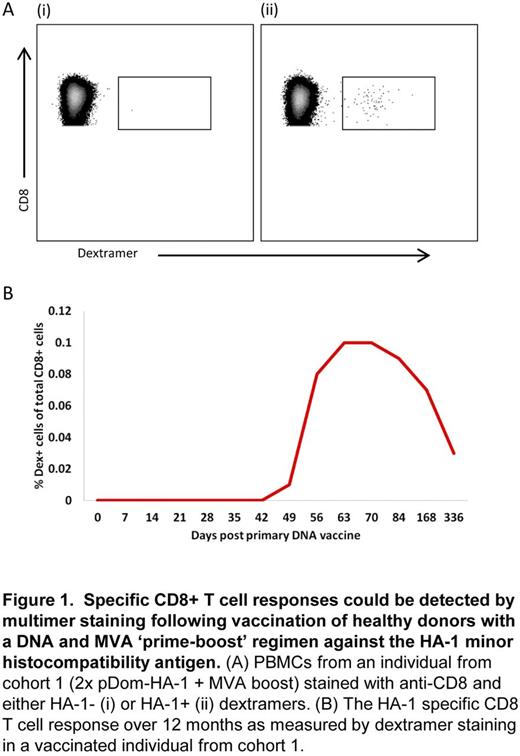
HA-1 specific CD8+ T cell responses were detectable by MHC-I multimer staining in 78% (7/9) of vaccinated individuals (Figure 1). Functional ex vivo responses to HA-1 peptide were confirmed byIFNγ ELISPOT assay. HA-1-specific responses were first detected between week 1 and 3 following MVA administration and displayed an effector memory (TEM) phenotype at the peak of the response, which transitioned to a more terminally differentiated TEMRA phenotype during 12 month follow-up. HA-1 specific CD8+ T cell clones analysed from 3 vaccinated individuals displayed polyfunctional cytokine profiles and cytotoxicity against HA-1 peptide-loaded targets.
CONCLUSION
We demonstrate that a heterologous prime-boost DNA-MVA vaccination strategy in HA1- healthy volunteers is tolerable and safe and elicits a detectable and sustained HA1-specific CD8+ T-cell response. 3 doses of 1mg pDOM-HA-1 with 1 dose of MVA-HA-1 boost is identified as the combination for evaluation in future Phase II studies. This demonstration of a vaccine-induced CD8+ T-cell response against an immunodominant mHAg provides the basis for trials to investigate the adoptive transfer of HA-1-specific CD8+ T-cells to recipients of allo-SCT as "vaccine-augmented DLI."
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.